
Talisman needle-free CGM demonstrates strong correlation to blood glucose in clinical studies and moves towards large volume manufacturing

/EIN News/ -- Talisman needle-free CGM demonstrates strong correlation to blood glucose in clinical studies and moves towards large volume manufacturing
- Data from Clinical Performance Study of Talisman shows strong correlation with blood glucose values
- GlucoModicum is partnering with world-class manufacturers to build large-volume production lines for Talisman devices and sensors
-
Recent completion of a funding round to enable the progression of manufacturing, clinical and regulatory approvals
Helsinki, Finland, 17 June 2024 – GlucoModicum, a company transforming glucose monitoring with proprietary needle-free magnetohydrodynamic (MHD) technology, today provides an update on the clinical performance, product development and commercialization pathway of Talisman, the Company’s needle-free continuous glucose monitor (CGM).
Talisman is a wearable CGM that uses MHD technology to extract interstitial fluid (ISF) from the skin without the use of needles. By integrating ultra-sensitive and low-cost biosensors, Talisman enables continuous, painless, and accurate monitoring of glucose levels.

Clinical Performance and manufacturing
Data from an ongoing Clinical Performance Study (FIMEA-K485) of Talisman, recently presented at the World Congress of Diabetes Technology and Therapeutics in Kochi, India, confirms a strong correlation to blood glucose values (currently, N>100 individual tests). The study, which commenced in 2023, will recruit up to 500 participants including patients with Type 2 diabetes and healthy volunteers and is authorized and monitored by the Finnish Medicines Agency (FIMEA) under EU regulation.
CLINICAL PERFORMANCE STUDY (FIMEA-K485)
ANALYSIS CORRELATION
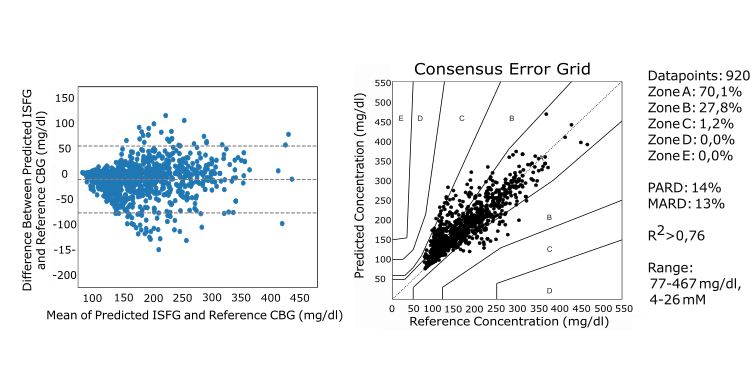
- Talisman yields strong correlation to reference blood glucose measurements in clinical performance studies.
- The study involves 100+ adult participants including persons with type 2 diabetes.
- The correlation and data obtained from the study serve to confer Talisman with the required accuracy.
Talisman, as being used in the CPS, is manufacturable in large volumes with low COGS. The device and sensor are nearing a “design-freeze”, enabling GlucoModicum to make progress in the manufacturing proof of principles and finalizing the design of automated high-volume manufacturing lines. The company will own the manufacturing lines and associated IPR and has received several offers for contract manufacturing from large world-class operators. The first automation line is designed to produce 21 million sensors annually which can be duplicated cost-efficiently with a 10-month lead time. For the device, the initial ramp-up volume will be 100 thousand devices annually, but the company has finalized the blueprints to be able to manufacture up to a million devices annually. The Company plans to enter a mass manufacturing service agreement (MSA) during 2024, anticipating device pilot production towards the end of 2024 and sensor pilot production in 2025. GlucoModicum remains compliant with the ISO 13485 standard in quality management, an important element in R&D and manufacturing partners’ operations.

Funding and progression towards launch
GlucoModicum recently completed a funding round backed by a consortium of international investors and family offices. In addition to private funding, GM has received a substantial amount of public (non-dilutive) funding with the option of receiving further public funding for manufacturing investments. Using this funding, the Company continues to make progress towards CE marking and FDA submission as it prepares for product launch, continuing its work with global key opinion leaders to evaluate and validate the Talisman, target patient segments, and develop use cases. GlucoModicum is in the process of designing pivotal clinical studies to be conducted in recognized research centers testing Talisman devices and sensors manufactured in the actual manufacturing lines as required by the regulations.
Alejandro García Pérez, Chief Technology Officer at GlucoModicum said: “We are thrilled by the positive results from the study, which highlight the significant advancements we have made in the development of Talisman as we approach its launch. GlucoModicum continues to progress across all the critical areas including manufacturing, R&D, and regulatory approval and we look forward to the accelerated path towards commercialization.”
Jokke Mäki, Managing Director of GlucoModicum, commented: “GlucoModicum is well funded to deliver its objectives and is supported by high-quality investors who share our vision of delivering a needle-free alternative to CGM for all. This represents a strong validation of our technology, and we look forward to accelerating our progress towards commercialisation while maintaining our strong track record.”
- END -
Contacts
ICR Consilium
Chris Welsh / Evi Useh
Email: glucomodicum@consilium-comms.com
About GlucoModicum
GlucoModicum is transforming glucose monitoring with precise, needle-free magnetohydrodynamic technology. Its proprietary platform has the potential to radically change how people monitor their health, creating solutions that are precise, accessible and needle-free, empowering people to live healthier lives. The company’s first product is a non-invasive, wearable glucose monitor for patients suffering from diabetes. GlucoModicum was founded in 2018 as a spinout of the University of Helsinki and combines an experienced, multi-disciplinary in-house team with world-class partners to deliver groundbreaking solutions for personal biomarker monitoring. www.glucomodicum.com
About Talisman
GlucoModicum’s magnetohydrodynamic (MHD) technology is currently being productized as the Talisman, the first new, truly non-invasive CGM technology. Talisman uses MHD to extract interstitial fluid (ISF) from the skin without the use of needles, enabling rapid, painless and accurate readings of glucose levels. Talisman addresses the needs of the ~500 million diabetics and ~500 million pre-diabetics globally, and serves as a tool to promote better health among the +2 billion people at risk of developing diabetes.
The effectiveness and accuracy of GlucoModicum’s technology has been demonstrated in peer-reviewed studies assessing the efficiency of ISF extraction, biosensor compatibility and correlation of readings to blood glucose levels.
https://doi.org/10.1038/s41598-021-86931-7
https://doi.org/10.1038/s41598-022-21424-9
https://doi.org/10.1016/j.bios.2022.114123

EIN Presswire does not exercise editorial control over third-party content provided, uploaded, published, or distributed by users of EIN Presswire. We are a distributor, not a publisher, of 3rd party content. Such content may contain the views, opinions, statements, offers, and other material of the respective users, suppliers, participants, or authors.


